-
NEUIGKEITEN
- EXPLORE
-
Blogs
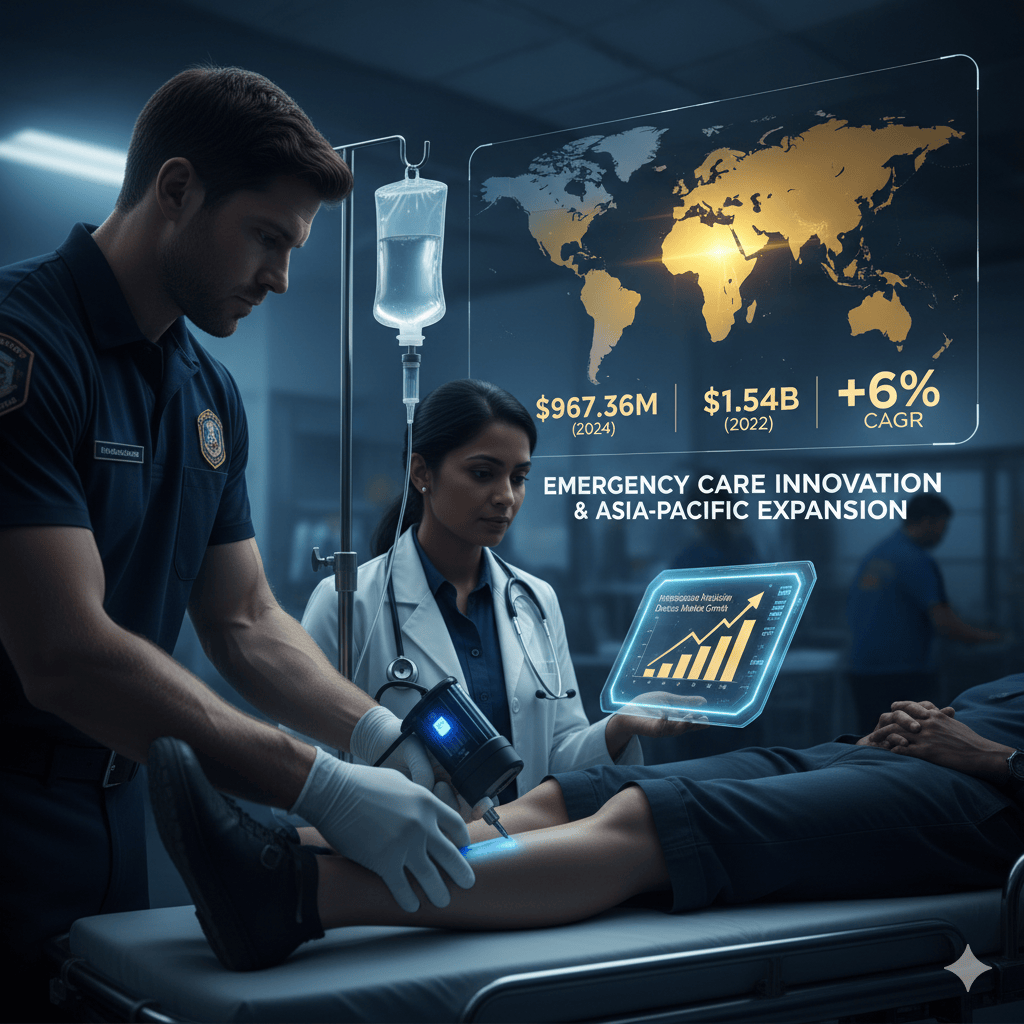
Global Intraosseous Infusion Devices Market Set for Significant Growth, Driven by Emergency Care Innovation and Asia-Pacific Expansion
The global healthcare landscape is constantly seeking faster, more reliable solutions for critical, life-saving interventions. Central to this pursuit is the Intraosseous Infusion Devices Market, which is experiencing robust expansion as a direct result of the urgent need for rapid vascular access in emergency and pre-hospital settings. According to detailed research compiled by Credence Research Inc., this vital market, which was valued at USD 967.36 million in 2024, is anticipated to reach USD 1,540.82 million by 2032, demonstrating a healthy Compound Annual Growth Rate (CAGR of 6%) during the forecast period (2024–2032).
Source: https://www.credenceresearch.com/report/intraosseous-infusion-devices-market
This comprehensive market growth is fundamentally propelled by the rising global prevalence of life-threatening conditions such as cardiac arrest, severe trauma, and shock, where conventional intravenous access is often difficult or impossible to secure quickly. The ability of intraosseous (IO) devices to deliver fluids and medications directly into the bone marrow providing a non-collapsible route to the central circulation positions them as indispensable tools in emergency care.
Market Drivers: The Core Need for Speed and Reliability
The primary catalyst for the Intraosseous Infusion Devices Market is the urgent demand for reliable vascular access in critical, time-sensitive situations. Every minute saved in administering life-saving drugs during trauma care or cardiac emergencies directly impacts patient survival rates. In these conditions, intraosseous infusion devices offer a safe, fast, and effective alternative to failed intravenous attempts, strengthening their adoption across hospitals and Emergency Medical Services (EMS).
Furthermore, advancements in technology have made these devices simpler to use and more reliable. Modern battery-powered devices and automated systems, such as Teleflex Incorporated’s Arrow EZ-IO system, are reducing the complexity of the procedure, ensuring consistent outcomes, and driving higher preference among healthcare professionals. Innovations in design focus on portability and ease of insertion, making them ideally suited for the demands of field operations.
A significant, concurrent market driver is the increasing adoption in military and disaster response settings. Portable systems, exemplified by the FAST1™ Intraosseous Infusion System, which is compact, lightweight, and offers high flow rates, are crucial for military medics and disaster relief teams operating in high-stress, resource-limited environments. Rising investments in comprehensive emergency care infrastructure and military medical systems across different regions further bolster the uptake of these essential tools.
Key Market Trends: Automation and Protocol Integration
The Intraosseous Infusion Devices Market is defined by two major trends: the growing integration of IO access into clinical protocols and a strong preference for user-friendly, automated technology.
1. Growing Integration into Emergency and Critical Care Protocols: Global clinical guidelines are increasingly recommending intraosseous access as a standard procedure when intravenous routes fail in critical cases. This procedural shift is driven by documented efficacy and a greater focus on pre-hospital care. EMS units, trauma centers, and hospitals worldwide are standardizing the use of devices like the EZ-IO, supported by thorough training programs to ensure proficiency among clinical staff. This integration ensures that reliable rapid vascular access is the norm, not the exception, in life-threatening scenarios.
2. Technological Advancements in Automated Systems: The preference is steadily moving towards automated and battery-powered devices over manual counterparts. These systems minimize the risk of user error and complications like bone fracture or incorrect placement, offering enhanced safety and efficiency. This focus on simplifying the procedure broadens the accessibility of the technology, especially in high-stress environments and emerging healthcare systems where advanced, specialized training might be less widespread. This continuous focus on innovation remains a key factor in strengthening device adoption globally.
Regional Dynamics: North America Leads, Asia-Pacific Fastest Growth
Geographically, the market presents a varied but promising outlook, as highlighted in the Credence Research Inc. analysis.
North America holds the largest market share, accounting for 42% in 2024. The region’s dominance is underpinned by advanced healthcare infrastructure, significant spending on medical services, and well-established, aggressive trauma care protocols. Widespread availability of battery-powered devices and routine integration of intraosseous access in both civilian and military medical units reinforce its market leadership.
Europe follows with a substantial 29% market share in 2024. The continent benefits from organized healthcare systems, strict medical standards, and supportive government initiatives that prioritize emergency preparedness and practitioner training. Countries with advanced trauma care infrastructure, such as the UK and Germany, are central to the region’s steady growth.
Crucially, the Asia-Pacific region is projected to be the fastest-growing regional market, securing a 21% share in 2024. This accelerating growth is fueled by rapidly expanding healthcare infrastructure, rising healthcare spending, increasing accident rates, and government-led initiatives to modernize emergency care. The region represents a major opportunity for manufacturers to increase market penetration, especially as awareness of advanced pre-hospital medical practices rises in high-growth countries like India and China.
Challenges and Opportunities: Cost vs. Accessibility
Despite the compelling growth trajectory, the Intraosseous Infusion Devices Market is not without its hurdles.
The most significant challenge remains the high costs of advanced automated systems, which often limit accessibility in developing regions and smaller, rural clinics. Coupled with the lack of favorable reimbursement policies in many emerging markets, this financial barrier restricts broader adoption.
Furthermore, the procedure’s dependency on proper, specialized training presents a challenge. Concerns over complications like infection or incorrect placement due to inadequate skills necessitate continuous, robust training and awareness programs to build clinical confidence and ensure patient safety.
However, these challenges frame significant market opportunities. The expanding applications across pre-hospital and emergency response systems from ambulances and air medical services to disaster relief offer a strong avenue for sustained demand. Simultaneously, innovation focused on developing cost-effective, user-friendly, and portable IO systems will open the door to widespread penetration in developing regions, strengthening the global market footprint.
Segmentation and Competitive Landscape
The market is segmented to reflect diverse clinical needs:
-
By Product: Dominated by Battery-Powered Devices (due to ease of use and consistency), followed by Manual Devices (for cost-sensitivity and portability) and Accessories (needles, stabilizers).
-
By Technology: Automated Systems hold a dominant share, preferred for their error reduction capabilities, while Semi-Automated Systems serve budget-conscious settings.
-
By End User: Hospitals remain the leading segment, with Emergency Medical Services (EMS) and the Military representing critical, fast-growing areas of adoption for field and pre-hospital use.
The competitive environment is robust, featuring key players like Teleflex, Inc. (with its EZ-IO system), Pyng Medical Corp., Becton Dickinson and Company, Cardinal Health, Inc., and Cook Medical Incorporated. Competition is centered on product reliability, the introduction of advanced, battery-powered and automated features, and strategic expansion into the rapidly growing Asia-Pacific and military medical sectors.
In conclusion, the Intraosseous Infusion Devices Market is positioned for a period of dynamic and necessary expansion. Driven by the non-negotiable requirement for rapid vascular access in time-critical emergency care situations and supported by ongoing technological advancements and strategic geographical shifts towards the Asia-Pacific fastest growth region, the market is fulfilling its crucial role as a core component of modern global trauma care and emergency preparedness. The data provided by Credence Research Inc. confirms that IO devices will continue to strengthen their integration into global emergency protocols, ensuring better patient outcomes worldwide.
Source: https://www.credenceresearch.com/report/intraosseous-infusion-devices-market