What Will Fuel the North America Cell-Free Protein Synthesis Reagents Market’s Rapid Growth to USD 292.22 Million by 2032?
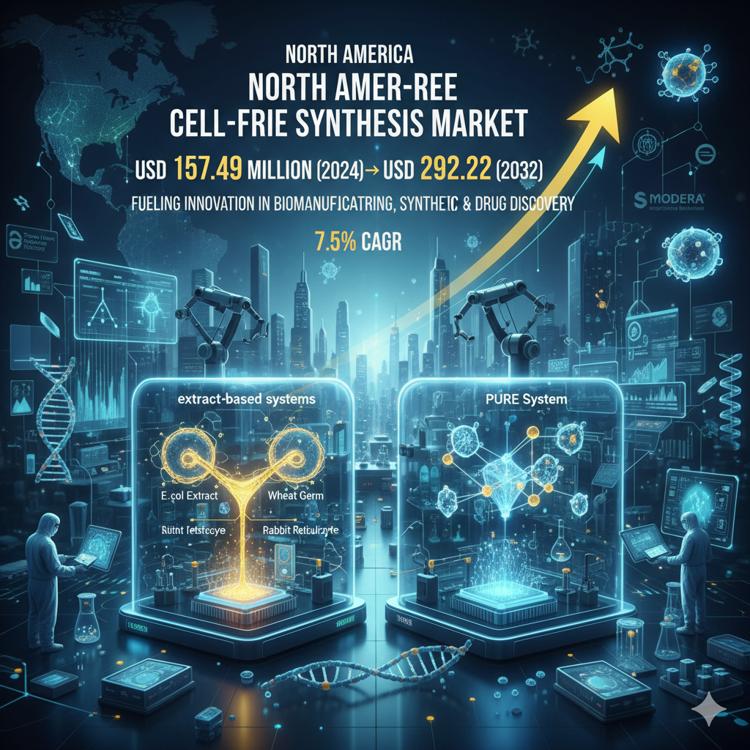
The North American life sciences landscape is undergoing a transformative shift, and one of the most compelling areas of this evolution is the cell-free protein synthesis reagents market. Valued at USD 157.49 million in 2024 and projected to reach USD 292.22 million by 2032, this segment is on a robust 7.5% CAGR trajectory, driven by innovations in biomanufacturing, synthetic biology, and high-throughput research applications.
Unlike conventional cell-based protein expression systems, cell-free platforms offer speed, flexibility, and scalability, making them a preferred choice for protein engineering, functional analysis, and drug discovery. As North America cements its global leadership in biopharmaceutical R&D, several converging forces are propelling this market toward remarkable growth.
In this article, we explore the key drivers, competitive dynamics, technological shifts, and application trends fueling the rapid rise of the North America cell-free protein synthesis reagents market.
1. R&D Leadership and Biopharma Infrastructure: The Core Growth Engine
The United States commands a 68% share of the regional market in 2024, reflecting its dominant position in biopharmaceutical research and development. Home to leading academic research institutions, federal funding initiatives, and advanced clinical trial infrastructure, the region provides a fertile ground for innovations in protein synthesis.
Top-tier organizations such as Thermo Fisher Scientific, Promega Corporation, New England Biolabs, Takara Bio, and Moderna Therapeutics leverage this environment to develop, scale, and commercialize next-generation reagents. These players are not only expanding their portfolios but also investing in automated, high-throughput systems that make cell-free protein synthesis more accessible to academic and industrial labs alike.
Additionally, emerging innovators such as Creative Biolabs, Sutro Biopharma, Arbor Biosciences, GeneCopoeia, BioCentury, Labscoop, and Tierra Biosciences are driving disruptive innovation in protein synthesis through specialized reagent systems and application-specific solutions.
This strong ecosystem of established leaders and agile newcomers creates healthy competition and accelerates the pace of technological advancement.
2. Technological Innovation: Extract-Based Systems Lead the Way
One of the defining features of the North America cell-free protein synthesis market is the dominance of extract-based systems, which hold a 72% market share. Systems derived from E. coli, wheat germ, and rabbit reticulocyte extracts are valued for their high yields, cost-effectiveness, and scalability.
These extracts have proven particularly effective in applications ranging from recombinant protein production to rapid functional screening, enabling researchers to bypass the complexities of cell culture. For example, Promega Corporation’s E. coli S30 Extract System for Circular DNA allows direct in vitro transcription and translation of genes from plasmid or lambda vectors, reducing turnaround time dramatically.
At the same time, reconstituted PURE systems are capturing a 28% market share. Though currently smaller, their adoption is growing in areas
1. Expanding Biopharmaceutical R&D Is Accelerating Market Demand
The rapid expansion of biopharmaceutical research is one of the strongest growth engines of the North American CFPS reagents market. Pharmaceutical and biotechnology companies are increasingly turning to cell-free systems to shorten development timelines and enhance innovation in therapeutic protein prototyping.
These systems enable rapid expression of proteins such as antibodies, enzymes, and vaccine components — critical for accelerating drug discovery and improving time-to-market for biologics. North America, particularly the United States, has a strong foundation to support this growth: a robust research ecosystem, top-tier laboratories, and abundant funding for advanced biopharmaceutical innovation.
· For example, GenScript introduced a commercial CFPS kit designed to accelerate therapeutic protein prototyping. The platform enables scientists to quickly test vaccine and antibody candidates without relying on complex cell cultures.
· The U.S. leads the region with a 68% share of the North American CFPS reagents market in 2024, largely due to advanced infrastructure and active investment in protein engineering research.
This alignment of R&D intensity and innovative tools is creating an ecosystem where CFPS is no longer a niche technology but a mainstream enabler of precision medicine and targeted biologics.
2. Rising Need for High-Throughput Screening Drives Rapid Adoption
Another key factor propelling market growth is the rising demand for high-throughput screening (HTS) — a technique that allows scientists to test thousands of protein or enzyme variants rapidly. Traditional cell-based expression systems often become bottlenecks in such workflows, but CFPS reagents eliminate these delays by allowing direct in-vitro expression and functional testing.
The North American market benefits from the strong adoption of automation and robotics, particularly in the pharmaceutical and academic sectors. This technological backbone enables seamless integration of CFPS into HTS pipelines for:
· Drug discovery
· Synthetic biology
· Functional genomics
· For example, New England Biolabs (NEB) reported a growing academic demand for its PURExpress CFPS system. Labs are using it to characterize enzymes at scale, accelerating synthetic biology research workflows.
· HTS combined with CFPS supports faster iteration cycles, lowering both the time and cost of experimental validation.
This surge in HTS-driven demand is helping transform CFPS reagents from specialized research tools into indispensable components of modern life sciences.
3. Flexibility and Scalability Give CFPS Platforms a Competitive Edge
A critical advantage of cell-free systems is their flexibility in producing proteins that are challenging or impossible to express in living cells — such as toxic, unstable, or membrane-associated proteins. This makes CFPS indispensable for applications ranging from enzyme engineering to vaccine development.
Moreover, these platforms support seamless scalability. Researchers can begin with small-scale experiments and expand to pilot or production scale without re-optimizing host systems.
North America’s established biomanufacturing infrastructure, strong supply chains, and the presence of leading reagent providers make it easier for laboratories and companies to integrate CFPS technologies into their workflows.
· Leading players such as Thermo Fisher Scientific, Promega Corporation, and Takara Bio offer CFPS solutions designed to meet both research and commercial manufacturing needs.
· Scalable CFPS platforms help companies reduce bottlenecks in therapeutic protein and industrial enzyme development, improving speed and efficiency.
This combination of flexibility, scalability, and industrial readiness positions CFPS as a strategic enabler of next-generation biologics.
4. Synthetic Biology Applications Are Creating New Market Opportunities
Synthetic biology — the engineering of biological components and systems for new functions — represents one of the most exciting growth frontiers for the CFPS reagents market. These reagents enable researchers to prototype gene circuits and biological pathways outside of living cells, dramatically increasing speed and control.
The United States has become a global hub for synthetic biology investments, with numerous startups, consortia, and government programs accelerating innovation in biomanufacturing.
· For instance, the U.S. Department of Energy Bioenergy Technologies Office committed over $178 million to synthetic biology and biomanufacturing R&D projects. Many of these initiatives use CFPS platforms to develop sustainable biofuels and biomaterials.
· CFPS reagents enable synthetic biologists to rapidly test metabolic pathways, helping bridge the gap between conceptual design and functional biological production.
With increasing demand for sustainable biomanufacturing, biofuels, engineered enzymes, and custom biomaterials, synthetic biology is expected to be a major long-term driver of market expansion.
5. Educational and Diagnostic Applications Broaden the Adoption Base
Beyond cutting-edge biopharma and synthetic biology, the CFPS reagents market is also growing through educational and diagnostic use.
· Universities and research institutes are integrating CFPS kits into teaching laboratories, giving students hands-on experience in molecular biology, biochemistry, and synthetic biology without the complexity of cell culture.
· Diagnostic companies are using CFPS reagents for rapid protein interaction assays, labeling studies, and assay development, particularly in point-of-care and low-resource settings.
· For example, Takara Bio offers lyophilized reagent kits that are easy to use and do not require cold-chain logistics. This lowers barriers for educational labs and small diagnostic firms, expanding market reach.
This democratization of CFPS technology builds a skilled future workforce and opens new commercial opportunities for reagent suppliers.
6. Strategic Ecosystem: A Mix of Industry Leaders and Innovators
The North America CFPS reagents market benefits from a diverse competitive landscape that blends established global life science leaders with innovative startups.
Key market players include:
· Thermo Fisher Scientific
· Promega Corporation
· New England Biolabs
· Takara Bio
· Moderna Therapeutics
· Creative Biolabs
· Sutro Biopharma
· Arbor Biosciences
· GeneCopoeia
· Tierra Biosciences
These companies compete on product innovation, high-throughput reagent systems, and strategic partnerships with academic and pharmaceutical research institutions. This healthy competition fuels continuous improvement, making CFPS technologies more efficient, affordable, and scalable.
7. Market Outlook: A Clear Path to USD 292.22 Million
The North America CFPS reagents market grew from USD 93.22 million in 2018 to USD 157.49 million in 2024 and is projected to reach USD 292.22 million by 2032.
Several factors underpin this sustained growth trajectory:
· Strong biopharmaceutical R&D spending in the U.S. and Canada.
· Widespread adoption of high-throughput technologies.
· Scalable CFPS platforms that reduce time and cost in biologics development.
· Expanding synthetic biology ecosystem and educational integration.
· Strategic investments by key industry players in infrastructure and product innovation.
These combined forces are reshaping the way proteins are designed, produced, and applied across industries.

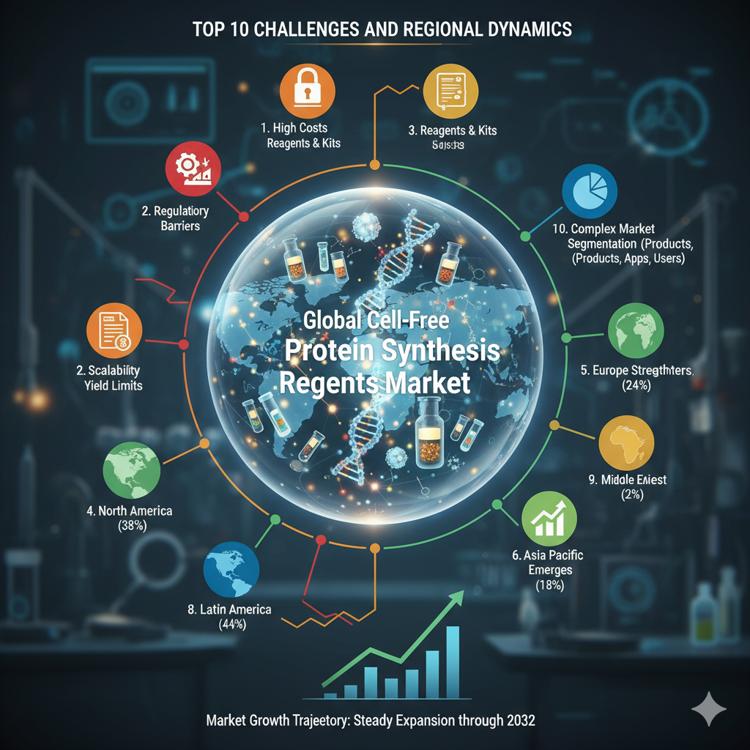
Top 10 Challenges and Regional Dynamics Shaping the Global Cell-Free Protein Synthesis Reagents Market
The global cell-free protein synthesis (CFPS) reagents market is on a strong growth trajectory, projected to expand steadily through 2032. Yet, alongside this upward curve, the industry faces significant cost, scalability, regulatory, and regional adoption challenges that must be addressed for the technology to reach its full commercial and research potential.
1. High Costs of Reagents and Kits Remain a Major Barrier
One of the most persistent obstacles in the CFPS landscape is the high cost of reagents and kits, especially PURE systems and lyophilized formats.
- These products rely on complex production of specialized enzymes and energy regeneration components, resulting in premium pricing.
- While pharmaceutical and biotech companies can typically absorb these costs, smaller research labs and academic institutions often face budgetary limitations.
- This cost imbalance restricts widespread adoption—especially in emerging markets and educational settings.
To overcome this, manufacturers must pursue cost-reduction strategies, streamlined production, and economies of scale to make CFPS more accessible.
2. Protein Yield and Scalability Constraints Limit Industrial Applications
Despite notable progress in CFPS technology, yield limitations remain a technical challenge.
- Extract-based systems often underperform when expressing complex proteins that require post-translational modifications.
- PURE systems, while cleaner, are expensive to scale, making them unsuitable for many therapeutic manufacturing applications.
- These challenges restrict CFPS to research, prototyping, and niche commercial uses, leaving a gap in industrial-scale protein production.
Improving reaction longevity, yield stability, and scalable production protocols will be essential for CFPS to compete with conventional cell-based expression platforms.
3. Regulatory and Standardization Barriers Delay Market Expansion
Regulatory uncertainty is another significant hurdle.
- The absence of harmonized protocols, validation frameworks, and industry-wide standards complicates CFPS integration into clinical and diagnostic workflows.
- Regulatory agencies demand reproducibility, safety, and quality assurance that current reagent systems may not fully deliver.
- Without clear regulatory pathways, companies face delays in scaling CFPS technologies for therapeutic and diagnostic use.
Establishing quality benchmarks, conducting clinical validations, and encouraging cross-industry collaboration will be critical to accelerating regulatory acceptance.
4. North America Leads — But Faces Cost and Capacity Pressures
- Market share: 38% in 2024
- Value: USD 157.49 million (2024), projected to reach USD 292.22 million by 2032 (CAGR 7.5%)
North America maintains a commanding lead in the global CFPS reagents market, driven by:
- Strong pharmaceutical R&D investment
- High adoption in biotechnology firms and academic institutions
- Presence of leading reagent suppliers such as Thermo Fisher Scientific, Promega Corporation, and Takara Bio
However, the region still faces cost pressures and scalability concerns, particularly for academic users. Addressing these will be crucial to sustaining its leadership position.
5. Europe Strengthens Its Position Through Synthetic Biology Clusters
- Market share: 24% in 2024
- Value: USD 99.54 million, expected to reach USD 173.51 million by 2032 (CAGR 6.6%)
Countries such as Germany, France, and the UK anchor Europe’s CFPS growth.
- EU-funded programs and biotech–academia collaborations play a major role in advancing synthetic biology applications.
- Demand is especially high for enzyme engineering and high-throughput screening workflows.
- Europe’s regulatory environment is more structured than many regions, which can accelerate standardization but also add compliance costs.
6. Asia Pacific Emerges as the Fastest-Growing Region
- Market share: 18% in 2024
- Value: USD 75.19 million, projected to reach USD 157.57 million by 2032 (CAGR 9.1%)
Asia Pacific is becoming a global growth engine for CFPS adoption.
- China, Japan, and India lead investments in biotechnology research and synthetic biology.
- Government funding and academic collaborations are expanding rapidly.
- Demand is increasing for affordable CFPS kits, making the region a critical target for reagent manufacturers seeking scale.
Despite infrastructure gaps in some countries, Asia Pacific’s momentum positions it as a key driver of future market expansion.
7. Latin America Shows Steady Growth Through Academic Integration
- Market share: 4% in 2024
- Value: USD 16.06 million, expected to reach USD 26.14 million by 2032 (CAGR 5.7%)
Latin America’s CFPS growth is concentrated in Brazil and Argentina.
- Universities and research centers are adopting CFPS for protein engineering and educational training.
- International collaborations with global reagent suppliers help bridge infrastructure gaps.
- Although growth is slower compared to developed regions, increasing biosimilar development is expected to drive steady demand.
8. Middle East Market Remains Niche but Promising
- Market share: 2% in 2024
- Value: USD 7.83 million, projected to reach USD 11.82 million by 2032 (CAGR 4.7%)
Growth is led by GCC countries and Israel, driven by:
- Expanding healthcare innovation ecosystems
- Investments in biotechnology startups
- Gradual integration of CFPS into enzyme engineering and diagnostic research
However, limited infrastructure and high reagent costs continue to limit scale. Future opportunities lie in targeted niche applications and public–private partnerships.
9. Africa Faces Infrastructure and Cost Barriers but Offers Long-Term Opportunities
- Market share: 1% in 2024
- Value: USD 4.53 million, projected to reach USD 6.06 million by 2032 (CAGR 3.1%)
- Adoption is currently centered in South Africa, where academic initiatives and diagnostic programs are growing.
- Budgetary constraints and limited lab facilities hinder broader use.
- Expanding STEM education programs and international partnerships with reagent suppliers can help unlock latent market potential in the region.
10. Complex Market Segmentation Reflects Diverse Adoption Pathways
The CFPS reagents market is segmented across multiple product, application, and end-user categories, which reflects its broad utility but also diverse adoption challenges:
By Product / Reagent Type
- Cell extracts and lysates (e.g., E. coli, wheat germ, rabbit reticulocyte)
- Transcription–translation mixes
- Amino acids & nucleotides, energy regeneration systems
- Enzymes & polymerases
- Kits & premixed consumables (including lyophilized formats)
- Accessories & consumables
By Method / System
- Extract-based systems
- Reconstituted PURE systems
By Application
- Protein engineering & functional analysis
- Enzyme engineering
- Therapeutic protein prototyping
- High-throughput screening & synthetic biology
- Protein–protein interaction & labeling studies
- Diagnostics & educational use
By End-User
- Pharmaceutical & biotech companies
- Academic & research institutes
- Contract research organizations (CROs)
- Diagnostics companies
- Educational laboratories
This segmentation illustrates how different user groups face different challenges—from regulatory hurdles for pharma to cost barriers for academia.
How Will Strategic Innovation and Competition Shape the Future of North America’s Cell-Free Protein Synthesis Reagents Market?
The North America cell-free protein synthesis reagents market is entering a defining growth phase, driven by intense competition, rapid technological progress, and strong R&D investments. As the industry races toward faster, more flexible, and scalable protein expression solutions, a single question stands out: how will the evolving competitive landscape shape the market’s future trajectory?
1. What Defines the Current Competitive Landscape?
The market is highly competitive, anchored by the presence of global leaders and regional innovators. Industry giants such as Thermo Fisher Scientific, Promega Corporation, New England Biolabs, and Takara Bio dominate through broad product portfolios, extensive R&D pipelines, and proven reliability. These companies focus on high-throughput kits, advanced enzyme formulations, and scalable solutions that meet the rigorous needs of pharmaceutical and academic research.
Meanwhile, emerging players—including Tierra Biosciences and Creative Biolabs—are intensifying competition by offering specialized synthetic biology and protein prototyping services. Their agility allows them to target niche applications and underserved segments, creating a dynamic environment where innovation moves quickly, and pricing pressure continues to rise.
This duality between established suppliers and nimble startups is accelerating technological advancement and ensuring that the market evolves rapidly.
2. How Are Companies Strengthening Their Market Positions?
Large players are betting on continuous product innovation and strategic collaborations to maintain their leadership:
· In June 2024, Daicel Arbor Biosciences launched its myTXTL Pro Kit and myTXTL Antibody/DS Kit, designed to speed up antibody discovery and protein engineering.
· In July 2024, LenioBio signed a supply agreement with Touchlight to integrate doggybone DNA (dbDNA™) technology, boosting rapid vaccine development for CEPI’s 100 Days Mission.
· In 2025, Thermo Fisher Scientific launched an optimized cell-free expression kit, reportedly improving protein yield by 20% across multiple biopharmaceutical applications.
· In January 2025, Sutro Biopharma entered a commercial-scale collaboration with Boehringer Ingelheim BioXcellence, leveraging Sutro’s modular cell-free platform.
Strategic alliances, licensing agreements, and co-development deals are central to maintaining competitive advantages. These moves not only expand market access but also accelerate the integration of cutting-edge technologies into real-world applications.
3. How Are Startups Reshaping Market Dynamics?
While the big players dominate infrastructure and global reach, startups are changing the game with cost-effective, customized, and education-focused solutions. Their offerings often prioritize:
· Affordable reagent kits for small labs and educational institutions.
· Specialized solutions for synthetic biology and protein prototyping.
· Faster turnaround times for R&D applications.
This grassroots expansion builds market depth, creating new customer segments beyond big pharma and research centers. It also exerts downward pricing pressure, forcing larger companies to innovate more efficiently.
4. What Strategic Factors Will Drive Future Growth?
The future outlook for the North America market is shaped by several interlinked trends:
· Rising Biopharma Investment: Increased funding in therapeutic protein development will expand demand for high-performance reagents.
· Synthetic Biology Expansion: Wider adoption of cell-free platforms in metabolic engineering, vaccine research, and biomaterials innovation will create new use cases.
· High-Throughput Integration: Automation and robotics will make large-scale screening faster and more accurate.
· Diagnostics Adoption: Cell-free systems will power rapid assays, accelerating diagnostic turnaround times.
· Education and Workforce Training: Affordable reagent kits will strengthen molecular biology training pipelines.
· Technological Evolution: Advances in PURE systems will improve protein purity and enable post-translational modifications.
· Collaborative Ecosystems: Partnerships between biotech firms and academic centers will speed up R&D.
· Startup Disruption: Affordable solutions from startups will expand accessibility and diversify the market.
· Supply Chain Strengthening: Addressing enzyme and nucleotide availability will enhance resilience.
· Innovation Hub: North America will remain the epicenter of global innovation, backed by strong infrastructure and funding.
5. How Will Technological Innovation Sustain Competitive Advantage?
Technological differentiation is the backbone of competition in this market. Players are focusing on:
· Optimized enzyme formulations to increase protein yield.
· Next-generation cell-free expression kits for simplified workflows.
· Integration with robotics to scale throughput without compromising accuracy.
· Modular and flexible systems that enable seamless scaling from R&D to production.
As demand for precision medicine, synthetic biology, and rapid vaccine development grows, the ability to innovate at speed will separate leaders from laggards.
6. What Role Will Collaborations Play in Market Acceleration?
Strategic partnerships are no longer optional—they are essential. Collaborations enable:
· Access to complementary technologies (e.g., DNA synthesis, robotics, and biomanufacturing platforms).
· Shared infrastructure and research funding, reducing time-to-market.
· Faster regulatory alignment, especially for therapeutic applications.
The Sutro–Boehringer Ingelheim BioXcellence collaboration is a prime example of how alliances can transform manufacturing capabilities and scale production of complex biologics.
7. How Will Market Competition Impact Pricing and Accessibility?
The presence of established global suppliers alongside cost-focused startups will create:
· Competitive pricing strategies aimed at maintaining market share.
· Wider accessibility for academic institutions and diagnostic firms.
· A more diversified market structure, supporting innovation across scales.
This healthy competition benefits end-users by improving product quality, expanding application areas, and driving down costs over time.
8. What Strategic Moves Should Companies Consider?
To thrive in this rapidly evolving landscape, companies should:
· Invest aggressively in R&D and product differentiation.
· Build strong partnerships with research institutes and industrial players.
· Focus on automation and high-throughput workflows.
· Offer tiered pricing models to cater to both enterprise and academic customers.
· Ensure supply chain resilience for critical reagents.
· Embrace regulatory preparedness for faster commercialization.

Conclusion:
In summary, the North America cell-free protein synthesis reagents market is projected to nearly double from USD 157.49 million in 2024 to USD 292.22 million by 2032, driven by a 7.5% CAGR. The market’s growth is underpinned by strong demand from pharmaceutical, biotechnology, and academic sectors, with established players like Thermo Fisher, Promega, NEB, and Takara Bio—alongside agile newcomers such as Creative Biolabs and Tierra Biosciences—shaping a highly competitive and innovation-oriented landscape. While extract-based systems currently command the majority share, increasing interest in PURE systems, high-throughput screening, and synthetic biology applications signal shifting trends. The United States leads the region with 68% market share in 2024, supported by deep biopharma infrastructure and research investment. However, challenges remain in reagent cost, yield limitations, regulatory standardization, and supply chain resilience. Looking ahead, the balance between technological breakthroughs, strategic collaborations, and cost efficiencies will determine which players climb to the top in this rapidly evolving market.







